What causes spinal muscular atrophy remains one of the most searched and discussed medical questions in the United States as genetic awareness, newborn screening, and early diagnosis continue to expand nationwide. This condition is now fully understood at a biological level, with no uncertainty about its origin. Medical science has clearly identified the internal mechanism that leads to the development of this neuromuscular disorder, allowing families to better understand why it occurs and how it progresses.
This in-depth report explains the confirmed origin of the condition, how it affects the body over time, why symptoms vary from person to person, and how modern medicine defines and approaches it today.
A Clear Overview of the Condition
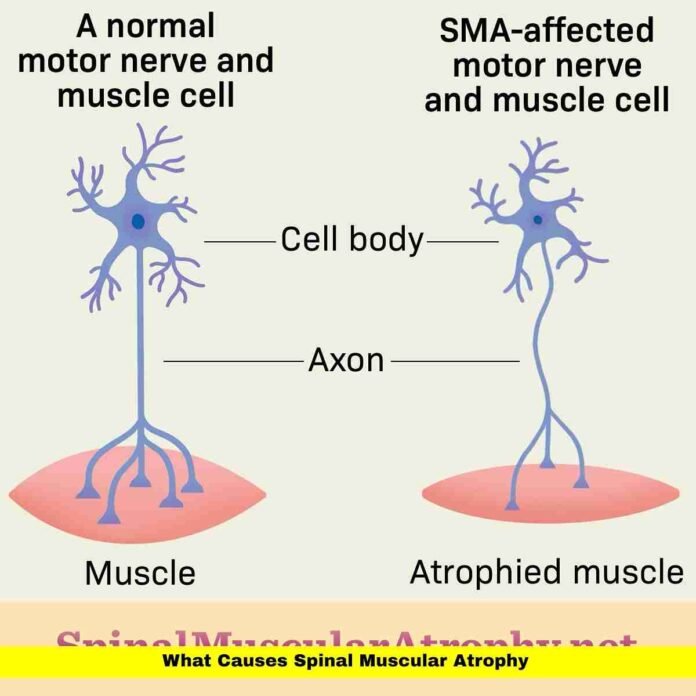
Spinal muscular atrophy is a genetic neuromuscular disorder that primarily affects voluntary muscle control. The condition targets motor neurons, which are specialized nerve cells located in the spinal cord and lower brainstem. These neurons are responsible for transmitting signals from the brain to the muscles, allowing actions such as sitting, walking, breathing, swallowing, and lifting objects.
When these motor neurons fail to function properly, muscles no longer receive consistent signals. As a result, muscles gradually weaken and shrink. This muscle wasting develops over time and can affect different parts of the body depending on severity.
Importantly, this condition does not impact intelligence, awareness, or sensory abilities. Individuals retain normal cognitive function, emotional development, and the ability to feel touch, pain, and temperature. The disorder is strictly related to muscle control.
The Genetic Origin Behind the Disorder
The root of spinal muscular atrophy lies in a specific genetic abnormality involving the SMN1 gene. This gene is essential for producing a protein that motor neurons need to survive. In healthy individuals, two working copies of this gene ensure sufficient production of the protein throughout life.
In affected individuals, both copies of the SMN1 gene are either missing or nonfunctional. Without these working copies, the body cannot maintain adequate protein levels. This shortage leads directly to the gradual breakdown and death of motor neurons.
This genetic change is present from conception. It does not develop due to illness, environment, lifestyle choices, or medical care during pregnancy or birth.
Why Motor Neurons Are Especially Vulnerable
Motor neurons are uniquely sensitive to reduced protein levels. The protein produced by the SMN1 gene plays a vital role in maintaining cellular stability within these neurons. It supports internal processes that keep nerve cells functioning efficiently and communicating properly with muscle fibers.
When protein levels fall too low, motor neurons experience internal stress. Over time, they lose their ability to repair themselves and eventually die. Once these neurons are lost, the body cannot replace them. This permanent loss explains why muscle weakness can worsen as time passes.
The selective vulnerability of motor neurons is one of the defining features of this disorder.
The Influence of the Backup Gene
Humans possess a second gene known as SMN2, which closely resembles SMN1. While this gene can also produce the necessary protein, it does so inefficiently. Most of the protein created by SMN2 is incomplete and breaks down before it can be used by motor neurons.
However, a small percentage of the protein produced by SMN2 is functional. The number of SMN2 copies a person has can significantly influence how much usable protein is available. This difference helps explain why symptoms can range from severe weakness in early life to milder muscle issues appearing later.
Although SMN2 cannot prevent the condition, it plays an important role in shaping how it presents.
How the Condition Is Passed Through Families
This disorder follows an autosomal recessive inheritance pattern. This means an individual must inherit two nonworking copies of the SMN1 gene to develop symptoms. Parents are often carriers, each having one faulty gene and one working gene.
Carriers are typically healthy and unaware of their status. A single working copy of the gene produces enough protein to maintain normal motor neuron function. Problems arise only when a child inherits the faulty gene from both parents.
This inheritance pattern explains why the condition can appear unexpectedly in families with no prior diagnosis.
Carrier Frequency Across the United States
Carrier status is relatively common in the U.S. population. Many people unknowingly carry one altered copy of the gene without ever experiencing symptoms. This widespread carrier frequency contributes to the ongoing occurrence of new cases each year.
Carrier screening is widely available and allows individuals to learn their genetic status. Such testing has become more common as awareness of inherited conditions grows.
Why Severity Differs Among Individuals
Although the genetic origin is the same for all affected individuals, symptom severity varies widely. This variation is largely linked to how much functional protein the body can still produce through backup mechanisms.
In more severe forms, motor neuron loss begins very early, sometimes before birth. In milder cases, neuron loss progresses slowly, allowing individuals to achieve milestones such as sitting, standing, or walking before weakness becomes noticeable.
Despite these differences, the underlying biological cause remains identical in all cases.
Understanding Early and Late Symptom Onset
The timing of symptom appearance depends on how quickly motor neurons are affected. In some individuals, muscle weakness becomes evident within the first months of life. In others, symptoms may not emerge until childhood, adolescence, or adulthood.
Motor neuron damage often begins before outward signs are visible. This delayed presentation explains why early identification is critical for preserving muscle function.
The Role of Newborn Screening
Newborn screening programs across the United States now include this condition as part of routine testing. This allows healthcare providers to identify affected infants before symptoms appear.
Early identification does not change the genetic origin, but it provides a critical opportunity for timely medical intervention. Preserving motor neurons early can significantly influence long-term physical outcomes.
Cases Without a Known Family History
In a small number of cases, the genetic change occurs spontaneously during the formation of reproductive cells. When this happens, there may be no known family history of the disorder.
Even in these rare situations, the internal mechanism remains the same. The absence of functional SMN1 gene copies leads to reduced protein production and motor neuron degeneration.
Progression Over Time
Spinal muscular atrophy is considered a progressive condition. Without sufficient protein support, motor neuron loss continues gradually. As more neurons are lost, muscle weakness becomes more pronounced.
The speed of progression varies, but the biological process itself remains ongoing unless protein levels are increased through medical intervention.
Why Thinking and Learning Remain Unaffected
The protein shortage primarily affects motor neurons. Neurons involved in memory, learning, and sensory processing are far less dependent on high protein levels.
As a result, individuals maintain normal cognitive abilities regardless of physical limitations. This distinction is consistently observed across age groups and severity levels.
Modern Treatment Approaches and Their Limits
Current FDA-authorized therapies focus on increasing protein availability within motor neurons. Some treatments address the missing gene function, while others improve how the backup gene produces protein.
These therapies do not alter inheritance patterns or eliminate the genetic change. Instead, they target the downstream effects that cause neuron loss. Earlier treatment generally leads to better preservation of muscle function.
The Importance of Genetic Counseling
Genetic counseling plays an essential role for individuals and families affected by inherited conditions. Counselors help explain inheritance patterns, carrier status, and future risk.
This guidance supports informed medical decisions and helps families understand complex genetic information in clear terms.
Dispelling Common Misconceptions
Despite growing awareness, misconceptions still exist. This condition is not linked to vaccinations, parenting choices, environmental exposure, or complications during delivery.
Understanding the true origin helps reduce stigma and misplaced guilt among families.
Why Awareness of the Cause Matters
A clear understanding of the biological origin empowers families to seek early testing, appropriate care, and long-term planning. Accurate information also supports public awareness and advocacy efforts.
As knowledge spreads, families are better equipped to navigate diagnosis and treatment with confidence.
Current Direction of Medical Research
Today’s research does not focus on redefining the cause. That aspect is firmly established. Instead, scientific efforts aim to improve long-term outcomes by preserving motor neurons and enhancing muscle strength over time.
This focus reflects confidence in the current understanding of the condition’s origin.
A Clear Summary of the Biological Cause
Spinal muscular atrophy results from the absence or malfunction of both copies of a critical gene needed for motor neuron survival. This leads to insufficient protein production, progressive neuron loss, and muscle weakness. Differences in severity arise from natural variations in backup genetic support, not from different causes.
Understanding the biological foundation behind this condition helps families feel informed and prepared—share your thoughts below or stay connected for the latest updates.